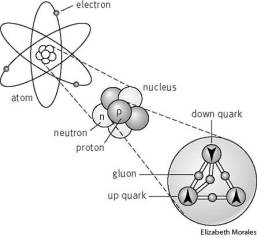
Statement: The Subatomic Particle Theory of matter represents a paradigm shift in science that occurred in the late 1800’s.
Analysis
Mankind has indulged in the process of acquiring new knowledge through curiosity, about the processes that go on around us and how could we use it for our benefit, needless to say humans have been quite successful in creating new scientific knowledge. Many examples exist that support this statement. Be it mastering metallurgy to sending humans on moon. But the journey has not been an easy one, It takes painstakingly long time to develop such knowledge and with many assumptions junked and new knowledge gained. It changed the way we look a the world. The subatomic particle theory is one such perfect example of how its discovery changed the way we did science. It was long thought that the atom was thought to be the smallest part of matter that could ever exist and the realm of chemistry became limited to that. But in the latter part of 1800’s, scientists discovered that atoms are composed of subatomic particles and that, no matter what the element, the same subatomic particles make up the atom. This helped tremendously in providing an explanation for the behaviour of the atoms. The Subatomic particle theory simply deals with the particles that are associated with an atom such as electrons, neutrons and photons, and how they behave. The discovery of the subatomic particles has aided in development of new technologies notable among them being the nuclear technology.
This was only due to our understanding of the way, atomic particles behaved and managed to manipulate them in the desired way.